Forschungsprofil Prof. Dr. Carsten Lüder
Die Arbeitsschwerpunkte der AG Lüder liegen auf der Interaktion von Toxoplasma gondii und seinen Wirten, insbesondere von Immunität und Immunevasion, Wirtszelltyp-spezifischen Reaktionen während der Infektion, und der Differenzierung unterschiedlicher Parasitenstadien. Unsere Arbeiten sollen helfen, zelluläre und molekulare Mechanismen aufzuklären, die einerseits zur Infektabwehr beitragen und schwere Krankheitsverläufe verhindern, und die es andererseits dem Parasiten ermöglichen, jahrelang persistierende Infektionen zu etablieren. Neuerdings interessieren wir uns außerdem dafür, wie sich die chronische Infektion auf die Immunreaktivität des Menschen auswirkt.
T. gondii ist ein weltweit vorkommender intrazellulärer Parasit von Menschen und Tieren; Schätzungen zufolge sind etwa 25-30% der Weltbevölkerung mit dem Parasiten infiziert. Infektionen verlaufen meist subklinisch, allerdings persistiert der Parasit in seinen Wirten lebenslang hauptsächlich in Gehirn und Muskulatur.

Personen mit Immunschwäche oder nach Infektion in utero sind dagegen von schweren Krankheitsverläufen bedroht. Aufgrund der hohen Prävalenz zählt T. gondii zu den wichtigsten Lebensmittel-übertragenen Erkrankungen des Menschen. Die Fähigkeit von Infizierten, den Parasiten meist zu kontrollieren, sowie die Fähigkeit der Parasiten zur lebenslangen Persistenz setzt eine gut regulierte Balance zwischen Erregervirulenz und Wirtsabwehr voraus. Die Erforschung dieser Interaktionen ist nicht nur ein faszinierendes Kapitel der Infektionsbiologie, sondern ist auch für die Entwicklung neuer Interventionsstrategien gegen die Toxoplasmose von Bedeutung.
Aktuelle Projekte
Inhibierung STAT-abhängiger Immunreaktionen
Interferon (IFN)-γ ist für eine effektive Immunabwehr von T. gondii essentiell. Es aktiviert den ‚signal transducer and activator of transcription‘ (STAT)-1, der in Makrophagen die Expression von etwa 1000 unterschiedlichen Genen reguliert, die u.a. für Moleküle der Infektabwehr, der Antigenpräsentation und der Immunregulation kodieren. In früheren Arbeiten haben wir gezeigt, dass T. gondii die Expression STAT-1-abhängiger Gene in Monozyten/Makrophagen weitgehend hemmt und der Parasit sich dadurch sein Überleben in IFN-γ-aktivierten Zellen sichert.

Neuere Arbeiten von uns und anderen Arbeitsgruppen zeigen, dass der Parasit die Bildung von Euchromatin durch Hemmung von Histonacetylierung und teilweise –methylierung hemmt und dadurch die Genexpression nach IFN-γ-Aktivierung verhindert. Außerdem führt er durch Bindung des Virulenzfaktors TgIST vor allem an STAT1-Tetramere zur Sequestrierung von STAT1 an funktionell irrelevanter DNA. Pharmakologisch konnten wir mittels dem Histondeacetylase-Inhibitor MS-275 zwar die IFN-γ-regulierte Genexpression in Gegenwart des Parasiten teilweise steigern, dies führte aber nicht zu einer verbesserten Parasitenabwehr.
Metabolische Regulation der Infektabwehr in Monozyten/Makrophagen
Monozyten und Makrophagen tragen als Wirtszellen während der akuten Toxoplasmose wesentlich zur Dissemination des Parasiten im Wirt bei. Als Abwehrzellen des angeborenen Immunsystems spielen sie auch eine zentrale Rolle in der Infektabwehr von T. gondii. Subpopulationen von Monozyten und Makrophagen unterscheiden sich funktionell deutlich, u.a. auch in der Infektabwehr von intrazellulären Pathogenen wie T. gondii. Unterschiedliche metabolische Eigenschaften wurden als wichtige Regulatoren der Infektabwehr erkannt (‚Immunometabolismus‘). Unsere bisherigen Arbeiten zeigen, dass die Glykolyse von Makrophagen die intrazelluläre Entwicklung von T. gondii kontrolliert. In diesem Projekt werden wir den Einfluss des Wirstzellmetabolismus auf die Infektabwehr von T. gondii untersuchen, und zugrundeliegende molekulare Mechanismen identifizieren.
Einfluss der Wirtszelle auf die Parasit-Wirt-Interaktion
T. gondii kann während der akuten Infektion durch aktive, parasiten-getriebene Invasion alle Kern-haltigen Zellen seiner Wirte infizieren. Der Infektionsverlauf in vivo wird daher wesentlich von den Interaktionen des Parasiten mit unterschiedlichen Wirtszelltypen bestimmt. Wir möchten verstehen, welchen Einfluss die zelluläre Mikroumgebung für die Interaktion von Parasit und Wirtszelle besitzt.

Durch duale ‚next generation‘ RNA-Sequenzierung haben wir in einem Pilotprojekt die Transkriptome von verschiedenen Wirtszelltypen vor und nach Infektion sowie die von T. gondii innerhalb dieser Zelltypen verglichen. Unsere Ergebnisse zeigen, dass verschiedene Zelltypen durch Hoch- und Runterregulation von Genen äußerst heterogen auf Infektion mit dem Parasiten reagieren. Auch T. gondii reguliert als Reaktion auf unterschiedliche Zellumgebungen die Expression verschiedene Gene. Unterschiedliche Wirtszelleigenschaften und die molekulare Flexibilität von T. gondii, auf zelluläre Nischen unterschiedlich zu reagieren, sind Gegenstand weiterer Arbeiten.
Stadienkonversion von T. gondii in Skelettmuskelzellen und Neuronen
Nach Erstinfektion disseminiert T. gondii in Form schnell replizierender Tachyzoiten. Innerhalb von 1 bis 2 Wochen differenzieren Tachyzoiten in langsam replizierende, weitgehend stoffwechselinaktive Bradyzoiten, die hauptsächlich in Neuronen und Muskelzellen persistieren und die chronische Infektionsphase charakterisieren. In diesem Projekt untersuchen wir die Hypothese, dass wirtszellspezifische Faktoren von Muskelzellen und Neuronen die Stadienkonversion von T. gondii fördern. Tatsächlich zeigen unsere bisherigen Arbeiten in einem in vitro-Modell mit Skelettmuskelzellen, dass terminal differenzierte, Zellzyklus-arretierte Myotuben, nicht aber deren proliferierende Vorläufer, sogenannte Myoblasten, die Bradyzoitenbildung fördern. Durch siRNA haben wir den Zellzyklusinhibitor Tspyl-2 als wichtigen Wirtszellregulator der Stadienkonversion von T. gondii identifiziert. Außerdem scheinen metabolische Eigenschaften der Myotuben, wie eine geringe Aktivität des Pentosephosphatweges, niedrige NADPH/NADP-Quotienten und erhöhte Mengen an reaktiven Sauerstoffspezies (ROS) eine wichtige Rolle zu spielen. Zurzeit etablieren wir auch ein Neuronen-Infektionsmodell, um in einem komplementären Forschungsansatz molekulare Wirtsfaktoren zu identifizieren, die die Stadienkonversion von T. gondii in Neuronen regulieren.
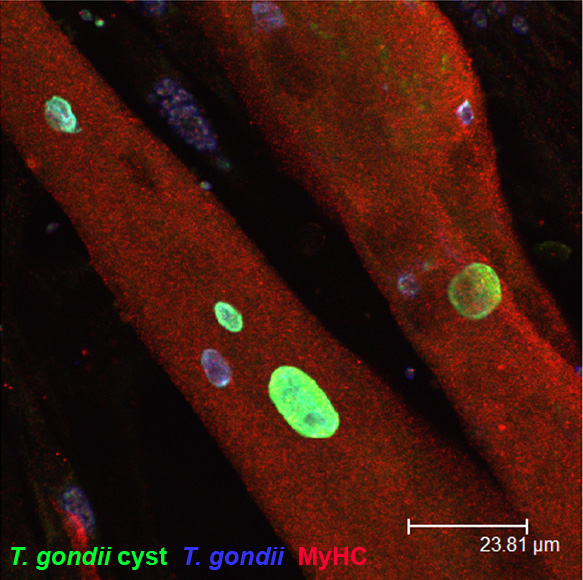
Einfluss chronischer Infektionen auf die Immunreaktivität
T. gondii führt in immungesunden Personen zu jahrelang persistierenden Infektionen. In Mäusen führen persistierende Toxoplasmosen zu verstärkten angeborenen Immunreaktionen, auch gegen heterologe Pathogene (‚trained immunity‘). Wir untersuchen, ob auch chronisch mit T. gondii infizierte Menschen phänotypisch und funktionell veränderte Immunreaktionen zeigen.
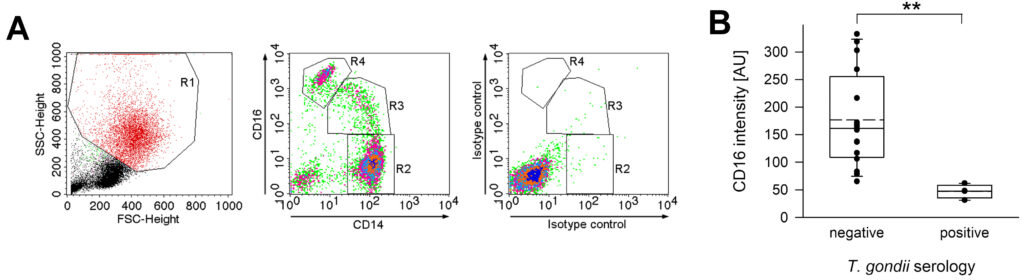
Unsere Arbeiten zeigen, dass Monozyten von gesunden Blutspender*innen, die chronisch mit T. gondii infiziert sind, weniger CD16 und CD62L, aber mehr CD64 auf ihrer Oberfläche exprimieren als Monozyten nicht-infizierter Kontrollpersonen. Außerdem reagieren sie nach Stimulation mit T. gondii in vitro mit einer stärkeren Expression von MHC Klasse II und dem Zytokin IL-12. Zurzeit untersuchen wir, ob weitere phänotypischen Eigenschaften von Monozyten durch eine chronische Toxoplasmose verändert sind, und ob sie auch auf heterologe Antigene anders reagieren als Monozyten von naïven Kontrollen. Dies hätte auch praktische Bedeutung, z.B. für Impfungen und Ko-Infektionen.
Mitarbeiter*innen & Alumni (letzte 5 Jahre)
Prof. Dr. Carsten Lüder (Projektleiter)
Cand. Dr. med. Vincent Buschatzky
Dr. med. Hauke Ehmen
Dr. med. Melanie Eisele
MSc (Microbiology, Biotechnology & Biochemistry) Hannah Fuchs
Cand. Dr. med. Hyeon-June Kim
Dr. rer. nat. Roswitha Nast
Dr. rer. nat. Md. Taibur Rahman
Cand. Dr. med. Noémie Thieffenat
Kooperationen
Dr. Martin Blume, Nachwuchsgruppe 2, Robert-Koch-Institut, Berlin
Prof. Dr. mult. Thomas Meyer, Molekulare Psychokardiologie, Universitätsmedizin Göttingen
Ausgewählte Publikationen
Rahman T, Swierzy IJ, Downie B, Salinas G, Blume M, McConville MJ, Lüder CGK. 2021. The redox homeostasis of skeletal muscle cells regulates stage differentiation of Toxoplasma gondii. Front. Cell. Infect. Microbiol., 11:798549. doi:10.3389/fcimb.2021.798549. [pubmed]
Nast R, Choepak T, Lüder CGK. 2020. Epigenetic control of IFN-γ host responses during infection with Toxoplasma gondii. Front. Immunol., 11:581241. doi: 10.3389/fimmu.2020.581241. [pubmed]
Ehmen HG, Lüder CGK. 2019. Long-term impact of Toxoplasma gondii infection on human monocytes. Front. Cell. Infect. Microbiol., 9:235. doi: 10.3389/fcimb.2019.000235. [pubmed]
Swierzy IJ, Händel U, Kaever A, Jarek M, Scharfe M, Schlüter D, Lüder CGK. 2017. Divergent co-transcriptomes of different host cells infected with Toxoplasma gondii reveal cell type-specific host-parasite interactions. Scientific Reports 7: 7229. doi:101038/s41598-017-07838-w. [pubmed]
Lüder CGK, Seeber F. 2016. Toxoplasma. In: Walochnik, J. & Duchene, M. (Eds.). Molecular Parasitology: Protozoan Parasites and their molecules. p. 217-239. Springer-Verlag, Wien, Austria.
Swierzy IJ, Lüder CGK. 2015. Withdrawal of skeletal muscle cells from cell cycle progression triggers differentiation of Toxoplasma gondii towards the bradyzoite stage. Cell. Microbiol.17, 2-17. doi: 10.1111/cmi.12342 Epub 2014 Sep 17. [pubmed]
Lang C, Hildebrandt A, Brand F, Opitz L, Dihazi H, Lüder CGK. 2012. Impaired chromatin remodelling at STAT1-regulated promoters leads to global unresponsiveness of Toxoplasma gondii-infected macrophages to IFN-g. PLoS Pathog. 8(1), e10002483. doi:101371/journal.ppat.1002483. [pubmed]
Hippe D, Weber A, Zhou L, Chang DC, Häcker G, Lüder CGK. 2009. Toxoplasma gondii infection confers resistance against BimS-induced apoptosis by preventing the activation and mitochondrial targeting of pro-apoptotic Bax. J. Cell Sci. 122, 3511-3521. [pubmed]
Lüder CGK, Stanway R, Chaussepied M, Langsley G, Heussler VT. 2009. Intracellular survival of apicomplexan parasites and host cell modification. Int. J. Parasitol. 39, 163-173. [pubmed]
Vutova P, Wirth M, Hippe D, Gross U, Schulze-Osthoff K, Schmitz I, Lüder CGK. 2007. Toxoplasma gondii inhibits Fas/CD95-triggered cell death by inducing aberrant processing and degradation of caspase 8. Cell. Microbiol. 9, 1556-1570. [pubmed]
Lüder CGK. 2007. Survival strategies of Toxoplasma gondii: Interference with regulatory and effector functions of macrophages. In: Denkers, E.Y. & Gazzinelli R.T. (Eds.). Protozoans in macrophages. p. 130-138. Landes Bioscience, Austin, TX, USA. ISBN: 978-1-58706-150-9.
Lüder CGK, Walter W, Beuerle B, Maeurer MJ, Gross U. 2001. Toxoplasma gondii down-regulates MHC class II gene expression and antigen presentation by murine macrophages via interference with nuclear translocation of STAT1a. Eur. J. Immunol. 31, 1475-1484. [pubmed]
Kontakt
Prof. Dr. rer. nat. Carsten Lüder, Tel. 0551-39 65869, Email: clueder(at)gwdg.de
